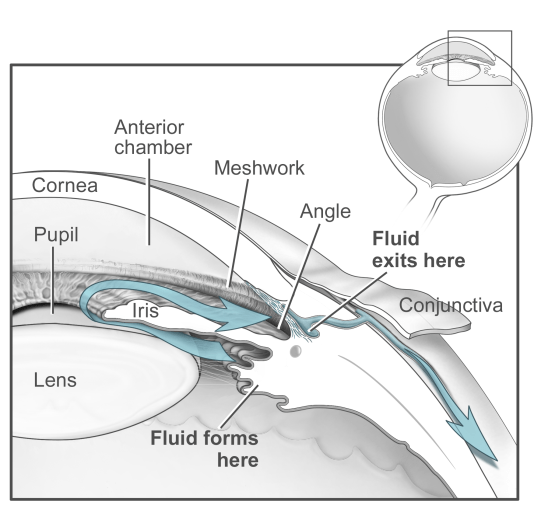
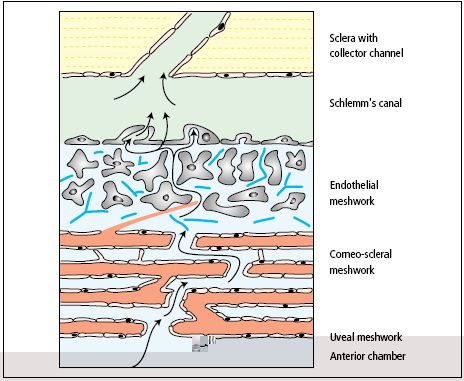
Glaucoma, one of the leading causes of blindness, impacts over 70 million people worldwide and is expected to continue to rise with an aging population [1]. The trabecular meshwork (TM), a specialized eye tissue located at the chamber angle of the eye next to the cornea (Fig. 1), regulates the outflow facility of the aqueous humor (AH) and controls the IOP (Fig. 2). In glaucoma, the resistance to aqueous humor outflow through the TM is increased thus elevating the intraocular pressure (IOP). However, the molecular mechanisms behind the increase in the resistance of outflow are not well defined. Currently, the Ethier lab is investigating in different directions to establish the relationship between molecular changes, IOP elevation and outflow resistance to understand the TM more clearly and test potential therapies to prevent IOP elevation and glaucoma development.
Figure 1: Schematic view of aqueous humor dynamics Figure 2: Schematic representation of trabecular meshwork [2].
(Source: National Eye Institute, National Institutes of Health)
A) Stem Cell Therapies for Trabecular Meshwork Regeneration (Eric Snider)
The cellularity in the trabecular meshwork has been shown to significantly decline with age, even more so in those with glaucoma [3]. This irreversible cell loss may be responsible for the increased IOP commonly seen in glaucoma. Consequently, restoring the function of the trabecular meshwork may halt glaucoma development entirely. Currently, no treatments exist that are capable of trabecular meshwork regeneration. In response, we are seeking to develop strategies to replenish the trabecular meshwork’s cellularity in hopes of lowering IOP and inhibiting the development of glaucoma. More specifically, we are investigating what potential stem cells may bring to restoring the cellularity of the trabecular meshwork. Depending on the naivety and source, stem cells are capable of differentiating to any cell in the body when exposed to certain stimuli. As a result, it may be possible to formulate an approach to push different stem cell populations toward a trabecular meshwork lineage.
B) Trabecular Meshwork Stiffness (Ke Wang)
The gene and protein expressions of trabecular meshwork cells are dependent upon the mechanical properties of the TM [4]. Abnormal deposition of proteins in the TM contributes to changes in outflow resistance and therefore elevation of pressure [5, 6]. We aim at characterizing the relationship between stiffness of TM and outflow facility of aqueous humor. We are seeking to develop methods based on atomic force microscopy to measure the local compressive modulus of the mouse TM. A computer controlled ocular perfusion system is used to measure the outflow resistance of enucleated mouse eyes [7].
- Quigley, H.A., Number of people with glaucoma worldwide. Br J Ophthalmol, 1996.80(5): p. 389-93.
- James, Bruce, and Anthony J. Bron. Ophthalmology: Lecture Notes. Chichester, West Sussex: Wiley-Blackwell, 2011. Print
- Alvarado, J., et al., Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci, 1981. 21(5): p. 714-27.
- Discher, D.E., P. Janmey, and Y.L. Wang, Tissue cells feel and respond to the stiffness of their substrate. Science, 2005. 310(5751): p. 1139-43.
- Tovar-Vidales, T., et al., Tissue transglutaminase expression and activity in normal and glaucomatous human trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci, 2008.49(2): p. 622-8.
- Knepper, P.A., et al., Glycosaminoglycans of the human trabecular meshwork in primary open-angle glaucoma. Invest Ophthalmol Vis Sci, 1996. 37(7): p. 1360-7.
- Lei, Y., et al., Outflow physiology of the mouse eye: pressure dependence and washout. Invest Ophthalmol Vis Sci, 2011. 52(3): p. 1865-71.